Imagine having all your data seamlessly integrated in one place! A recent Oracle survey revealed that 50% of clinical data managers rely on up to five different data sources, making it challenging to draw meaningful insights from trial data. The complexity of managing multiple platforms creates data silos, isolating information and making it difficult for data managers to achieve a comprehensive view. This fragmentation not only increases the risk of data inconsistencies but also slows down the decision-making process, hampering the overall efficiency of clinical trials.
Beyond data consolidation challenges, the use of disparate systems leads to broader operational complications. Disparate solutions designed for specific tasks are often not scalable or adaptable to new technologies, requiring costly modifications to remain compliant with evolving regulations. Additionally, managing communication through unsecured methods like email increases the risk of data privacy issues—a critical concern in today’s digital transformation in healthcare, and corporate cross-contamination. This fragmented user experience undermines operational quality and increases inefficiencies, as more time spent on management and training drives up costs and reduces trial management effectiveness. However, with Cloud Concinnity®, costs could potentially be reduced, leading to a 30-50% ROI and enhancing overall efficiency.
The pharmaceutical industry is moving away from traditional point systems—discrete tools designed for individual tasks—towards centralized cloud platforms. These unified platforms centralize all trial-related data, eliminating inefficiencies and improving data integrity. This shift represents not just a technological upgrade but a fundamental rethinking of how trials are managed, offering the potential to enhance overall research quality.
Cloud Concinnity® is at the forefront of this transformation, serving as a centralization hub for safety review activities. By offering streamlined access to essential tools from any location, Cloud Concinnity effectively resolves the complexities of managing multiple disparate systems. Its key features include:
Automated Task Alerts: Keeps teams aligned with timely reminders, ensuring tasks are completed efficiently and on schedule
Time-Saving Safety Listings: Streamlines safety data review, reducing manual effort and speeding up the process
Document Distribution via Standard Workflows: Automates the distribution process with consistent workflows, minimizing administrative workload
Role-Based Access Control: Ensures secure access control and seamless user transitions while minimizing data breach risks. Cloud Concinnity also includes a 21 CFR Part 11 compliant login with 2-factor authentication for enhanced data security
Robust Notification Center: Sends automated reminders to keep teams informed, enhancing coordination across dispersed teams
Centralized Dashboard: Provides a real-time overview of all studies and processes
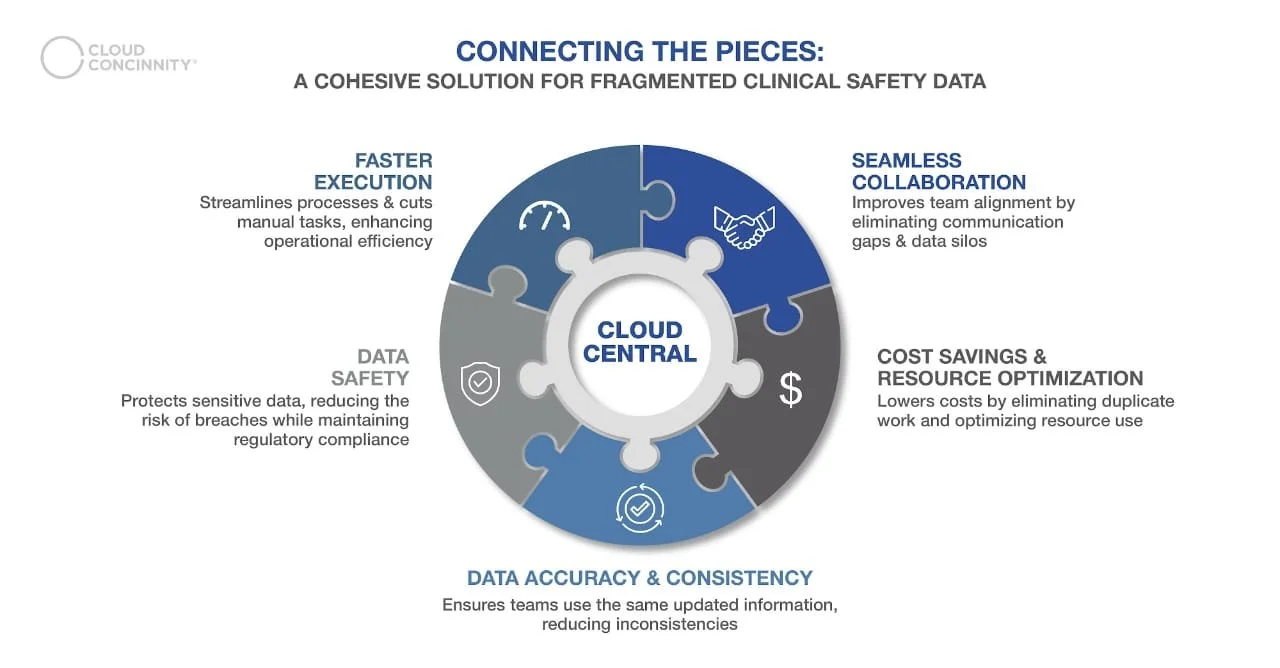
Cloud Concinnity’s centralizing features offer several overarching benefits, like.
Seamless Collaboration: Teams across different locations can collaborate in real time with unified access to up-to-date data, speeding up decision-making and fostering greater coordination
Data Accuracy and Consistency: Centralized management eliminates redundancies and ensures data integrity, so every site operates on accurate, updated information without duplication or confusion
Data Safety: By mitigating breach risks and maintaining regulatory compliance, Cloud Concinnity safeguards sensitive information, ensuring secure data handling throughout the trial process
Faster Execution: With automated alerts and faster safety reviews, Cloud Concinnity reduces the time spent on manual tasks, accelerating the clinical trial process
Cost Savings and Resource Optimization: By reducing administrative burdens and avoiding duplicative work, Cloud Concinnity helps streamline operations, ensuring that trials remain on budget while improving overall outcomes
There has always been a clear need for centralized platforms in clinical safety review processes, but sponsors were hesitant to adopt them due to various factors, particularly data privacy concerns. Impact of COVID-19, however, drastically changed this perspective. With social distancing and remote work becoming widespread, the limitations of fragmented systems became apparent. Centralized platforms quickly emerged as the more reliable and efficient solution, offering streamlined data handling, and a cost-effective approach. As a result, leveraging centralized technologies has become critical for the future success of clinical trials.
Looking ahead, the future of clinical research will be increasingly shaped by the integration of advanced technologies, with AI in Healthcare and machine learning playing a central role. These advanced technologies will enable automated data quality checks, statistical analysis, and visualization techniques, rapidly identifying patterns, inconsistencies, and missing data. Centralized platforms, powered by AI and automation, will play a pivotal role in making clinical trials faster, more reliable, and more secure, ensuring that research adapts to the growing complexities of the healthcare landscape.