Breast cancer remains the most prevalent cancer worldwide, with approximately 2.3 million new cases diagnosed each year. In the US, about 1 in every 8 women will be affected by breast cancer in her lifetime [1]. As researchers push the boundaries of innovation to develop better treatment options, clinical trials play a pivotal role in advancing groundbreaking therapies. However, navigating the regulatory landscape of breast cancer trials is a complex process. These trials must adhere to rigorous regulatory frameworks to ensure patient safety, data integrity, and scientific validity and must comply with strict regulatory frameworks to ensure patient safety, data integrity, and scientific validity. Ensuring compliance with these stringent regulatory requirements remains a significant hurdle, yet it is crucial for the success of these trials.
This article delves into the challenges faced in regulatory compliance of breast cancer trials, explores opportunities to enhance the process, and highlights how we support efficient and compliant clinical trial oversight.
Recent Innovations in Breast Cancer Clinical Trials
Recent innovations in breast cancer clinical trials focus on improving detection, diagnosis, monitoring, and treatment using new technologies, drug combinations, and personalized approaches driven by clinical trials. Some key innovations include:
Immunotherapy in Triple-Negative Breast Cancer (TNBC): A major study is evaluating whether some patients can stop immunotherapy after surgery if all signs of cancer are gone. This could help reduce side effects while still preventing the cancer from coming back[2].
Label expansion for Enhertu (trastuzumab deruxtecan): Enhertu was approved for HER2-positive breast cancer, but this new indication extends its use to patients with HER2-low and HER2-ultralow metastatic breast cancer also, a group that previously had limited targeted treatment options[3].
Precision Medicine for Breast Cancer: New treatment combinations are making cancer care more personalized. These include special drugs that target cancer cells in different ways, improving treatment for patients with tough-to-treat breast cancers like TNBC[4].
Tumor Profiling: Doctors are using tumor profiling, looking for certain genes within the tumor to help select the most appropriate treatment path. Looking at the genetic profile of the tumor can help predict whether the cancer is likely to reoccur or metastasize and whether chemotherapy is recommended in patients with early-stage, hormone receptor-positive breast cancer[5].
3-D Mammography The Tomosynthesis Mammographic Imaging Screening Trial (TMIST) compares the number of advanced cancers detected in women screened with 3-D mammography to those detected in women screened with 2-D mammography[5].
New Imaging Tests: Newer types of tests are being developed for breast imaging, including Scintimammography (molecular breast imaging), Positron emission mammography (PEM), Electrical impedance imaging (EIT), and Elastography[5].
Challenges in Regulatory Compliance for Breast Cancer Trials
Breast cancer clinical trials face numerous regulatory hurdles that can delay drug approvals which further increase costs, and impact patient access to innovative treatments. So let’s take a closer look at these challenges.
Regulatory Barriers and Approval Delays: Differences in regulatory requirements between agencies like the US FDA and the EMA can lead to approval delays due to a lack of harmonization, making clinical trials more expensive and posing significant financial barriers. This, in turn, makes it increasingly challenging to bring new drugs to market[6].
Post-Approval Change Management: Managing changes post-approval is crucial but faces regulatory obstacles, such as issues with dossier preparation and data[6].
Surrogate vs. Event Endpoints: Surrogate endpoints in oncology allow faster assessments and FDA approval but require careful validation. Without follow-up studies, their correlation with survival may be uncertain[6].
Opportunities in Regulatory Compliance for Breast Cancer Trials
As breast cancer research advances, regulatory frameworks must evolve to keep pace with innovation. Streamlining compliance processes can accelerate approvals, improve global collaboration, and enhance patient access to breakthrough therapies. Let’s explore some key opportunities shaping the future of regulatory compliance.
Harmonization of Regulatory Requirements: Harmonizing regulatory requirements across different countries can reduce duplication of effort for pharmaceutical companies, increase efficiency, improve access to innovative treatments, and facilitate global trials[6].
Agile and Responsive Regulation: Regulators can adopt risk-proportionate approaches to reviewing clinical trial applications, which can expedite the decision-making process[7].
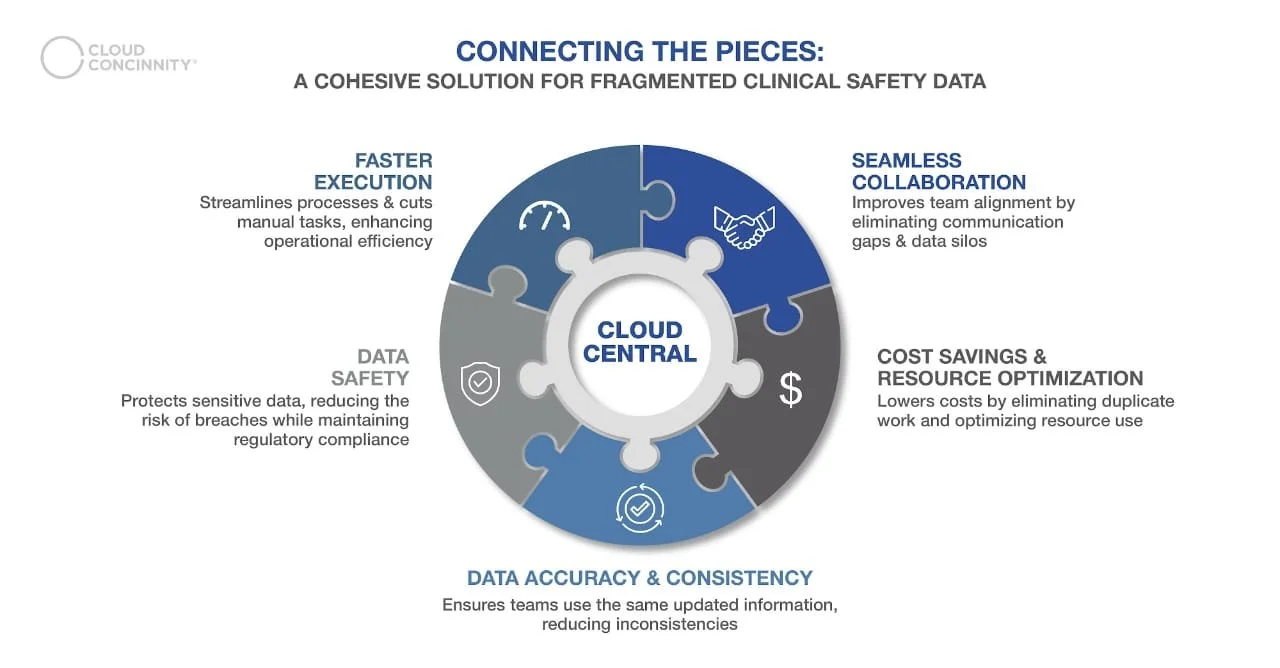
Leveraging Cloud-Based Platforms for Regulatory Management: Cloud-based platforms offer an accessible solution for managing data, streamlining workflows, and enhancing collaboration. However, Cloud Concinnity goes beyond protecting sensitive data, training users, and managing studies. It streamlines clinical oversight with unified account management, controlled document collaboration, centralized alerts, compliance monitoring, automated audits, flexible workflows, and adaptive pricing.
Usage of Accelerated Approval Pathways: Utilizing accelerated approval pathways with predefined surrogate endpoints can help avoid delays in the approval of innovative breast cancer therapies[6].
Real-World Data (RWD) Integration: Utilizing real-world data alongside clinical trial data can strengthen regulatory submissions, demonstrating the real-world effectiveness of new treatments.
Cloud Concinnity’s Unified Platform: A Game Changer
Navigating regulatory compliance in breast cancer clinical trials is a challenging yet rewarding endeavor. Adopting cutting-edge technologies and strategic approaches can transform compliance into a competitive advantage as the landscape evolves with new innovations and global harmonization efforts. At Cloud Concinnity, we are committed to driving efficiency, transparency, and innovation in clinical trial oversight.
Here’s how we can help:
Centralized and Compliant Hub: Our cloud-based platform offers a secure, unified space for managing trial documentation, approvals, and regulatory updates.
Facilitated Access & Collaboration: We provide seamless access to essential documents, enabling teams to collaborate effectively while maintaining compliance.
Automated Risk Mitigation: Our system automates compliance tracking, reducing the likelihood of regulatory breaches and improving trial efficiency.
Enhanced Transparency & Reporting: With real-time dashboards and automated reporting, Cloud Concinnity ensures that trial sponsors and regulatory bodies have full visibility into
Click here to explore how Cloud Concinnity can help your breast cancer trial meet regulatory standards with ease.