Bringing Good Process to Clinical Trial Oversight
The future of clinical trial oversight is distinctly digital. In the wake of COVID-19, clinical trials are embracing a new normal that will reach far beyond the pandemic. As this new era dawns, the entire clinical trial ecosystem continues to wrestle with a multitude of challenges. The new era of clinical trial oversight will be powered by smart software.
Clinical trial oversight committees need the best possible tools to address today’s challenges. That’s why Cloud Concinnity’s single, secure cloud-based hub provides rapid data access for smoother collaboration, real-time communication for empowered decision-making, and secure visibility for efficient organization.
THE OVERSIGHT ECOSYSTEM. SIMPLIFIED WITH A SINGLE, SECURE HUB
Cloud Concinnity® delivers:
Shortened time to market for treatments to save lives, accelerate revenue
Mitigation of litigation risk & reduction of legal expense
Elimination of burdensome administrative & operational complexity
1. FACILITATED ACCESS
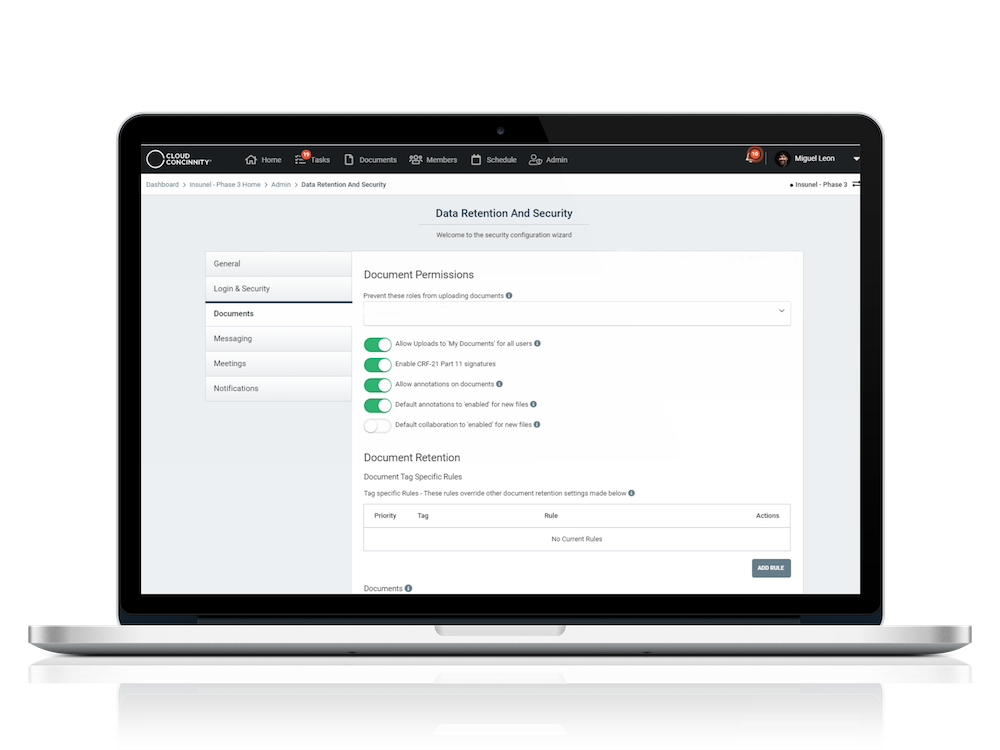
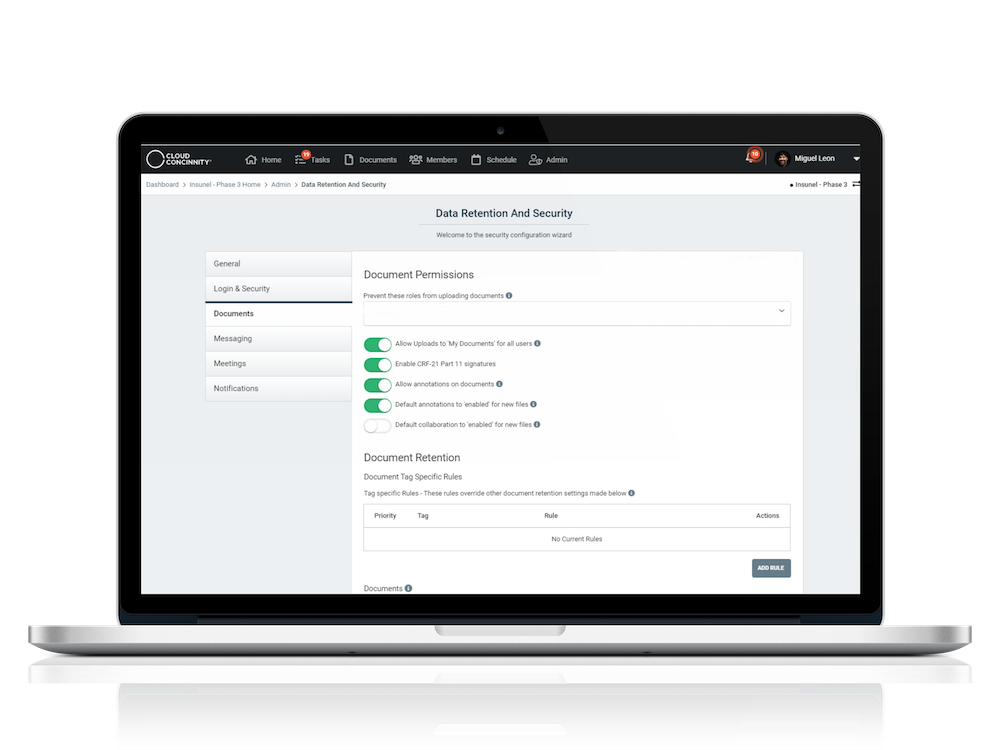
As the digitization of clinical trials continues, changes in how study data is captured and recorded will have a profound impact on how oversight committees review data. As a hub for secure, full-featured interaction, Cloud Concinnity® delivers simplified access for the new clinical trial governance and oversight ecosystem.
INFORMATION MANAGEMENT
Dual workspaces, including one Blinded Workspace and one Unblinded Workspace for each study
Role-based permissions ensure unblinded data security
Automated push notifications for timely responses
Full-featured document library with drag & drop uploading, smart tagging and version control
Built-in templates for agendas, minutes & important documents
Dashboards provide easy access to high priority study materials
Finalized & signed documents can be tagged to the eTMF for easy availability as PDFs
COMMUNICATION TOOLS
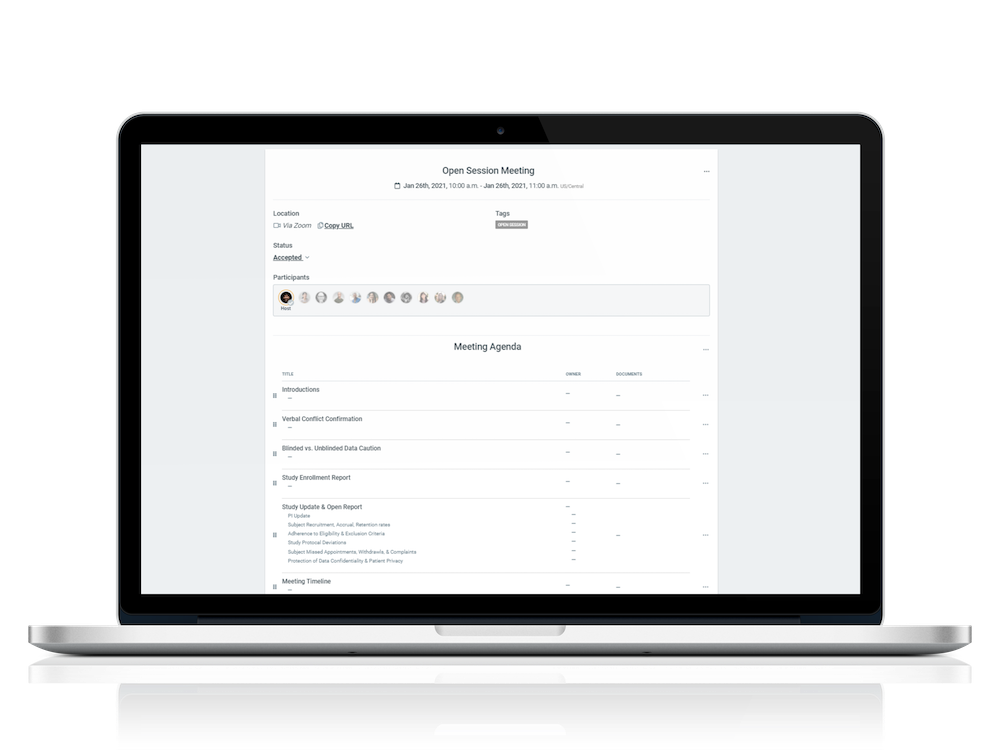
Integrated meeting features support seamless video conferencing
Meeting features include agenda templates, document attachments, voting tools, customizable surveys, and recording of minutes for both open and closed sessions
Robust messaging and voice capabilities for secure communication with group and direct messaging
Time sensitive reminders and alerts remove friction from key activities
DATA INTEGRATIONS
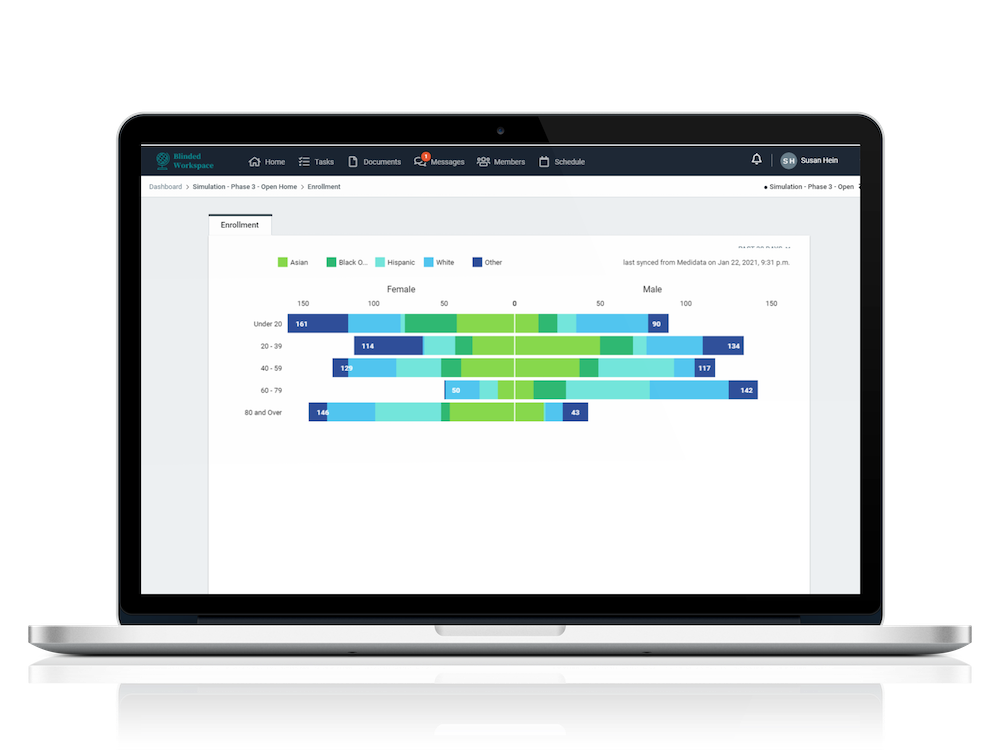
EDC integration (Medidata, Medrio, RedCap Cloud) allows for seamless reporting of important information like adverse events, enrollment and more
Docusign enables 21 CFR Part 11 compliant signatures
Microsoft Office 365 and Box integrations enable annotations and comments
Zoom integration powers seamless video conferencing from the Zoom app or web browser
2. CONTROLLED PROCESS
Cloud Concinnity® brings every interaction online, streamlining the costly and time-consuming work of scheduling and sharing work across platforms and time zones. Oversight committees will have more time to think, plan for and attend to their substantive, complex demands.
WORKFLOW AUTOMATION
Out-of-the-box workflows bundle every action required to complete key oversight responsibilities
Automated alerts mean no tasks get lost
Integrated reporting tracks each task & step
Consistent application of best practices will significantly reduce risk of delays, errors and noncompliance
Ability to efficiently manage multiple workflows over multiple studies scales operations
Workflow progress reporting elevates visibility and reduces delays
COLLABORATION
Vital communication positioned alongside research, study data and to-do’s saves time
Sponsors, CROs, and DMC members can stay in touch as needed, not just during scheduled meetings
Agenda builder, scheduling & calendar tools smooth out complicated collaboration
Dual workspaces for controlled studies allow for smoother collaboration of players within the DMC ecosystem without risk of unblinded data breaches
MEETING MANAGEMENT
End-to-end meeting workflows eliminate countless emails and time-consuming delays
Meetings can be hosted on the platform’s secure video conferencing, with all relevant material side by side
Draft and finalize meeting minutes, then prepare for e-signature, which is integrated into the system
All of a participant's meetings and tasks, across multiple studies if applicable, will sync into a single Global Calendar
3. TRANSPARENT OUTCOMES
Cloud Concinnity® brings every interaction online, streamlining the costly and time-consuming work of scheduling and sharing work across platforms and time zones. Oversight committees will have more time to think, plan for and attend to their substantive, complex demands.
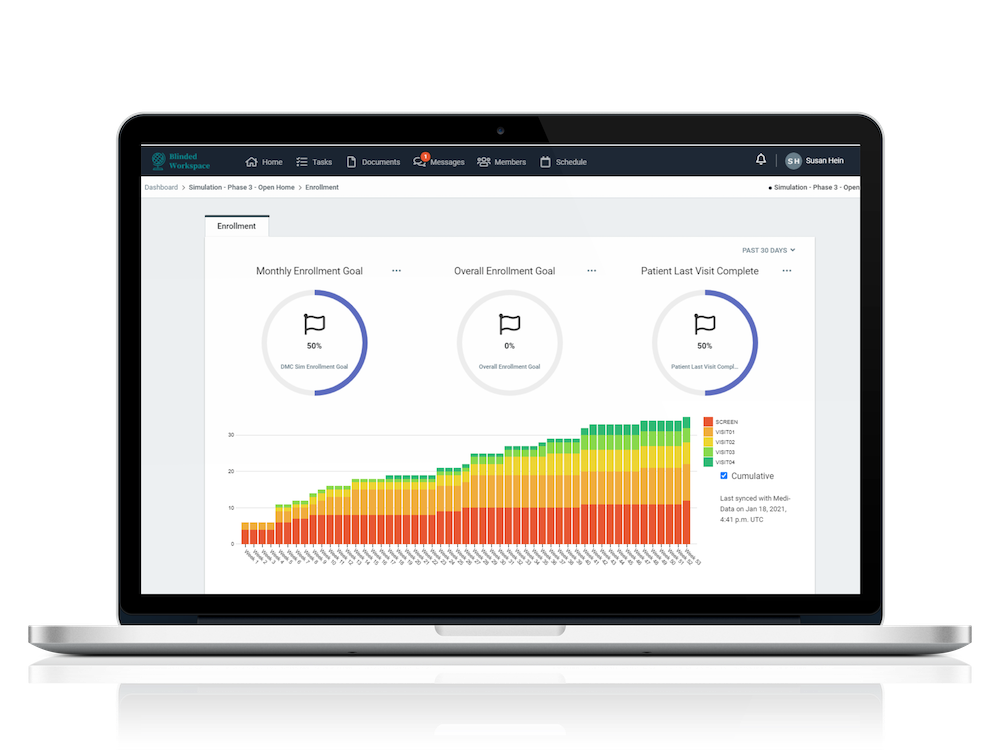
MASTER DASHBOARD & ANALYTICS
Master dashboard where up to date information on multiple studies can be reviewed 24x7
Unprecedented visibility into individual study results and key metrics on a customizable study dashboard
Track and measure a multitude of metrics and activities with robust, easy to use reporting
A single, secure hub for the aggregation, review and submission of data for sponsors & regulators
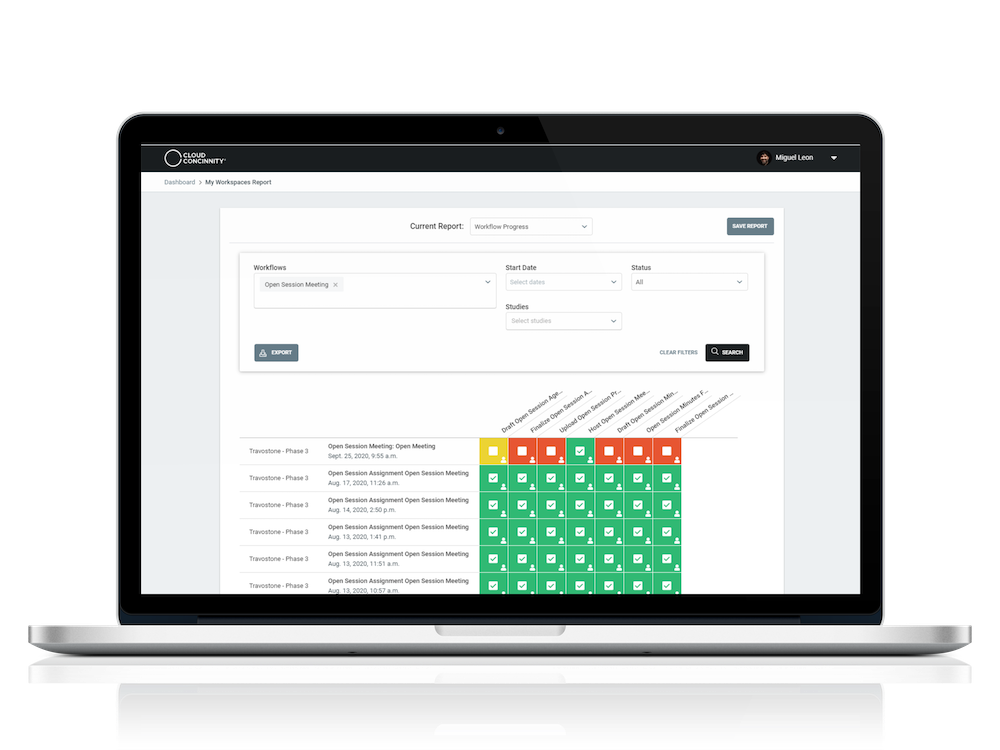
WORKFLOW PROGRESS REPORTS
Workflow progress reports provide anAdministrator visibility into DMC activity at the study or multi-study level
Role-based and individual task assignments with appropriate levels of automation
Full visibility into the status of each workflow to eliminate bottlenecks and keep the process moving forward
AUDIT-READY INFORMATION OUTPUT
System has been designed with push-of-a-button access to information required for internal or external audit purposes
Audit-ready tracking of documents, tasks, messages, meetings, minutes, role changes and more
Data retention feature allows secure storage for as long as desired by the sponsor
Easy to use tools for reviewing and submitting TLFs
Use standard or custom templates for creating eTMF and related FDA documentation
Easy delivery of zipped folder of final eTMF (Trial Master File) documentation
4. VERIFIED SECURITY & REGULATORY COMPLIANCE
Powerful security and regulatory compliance features are critical for today’s clinical trial oversight software. Because Cloud Concinnity® was originally developed to provide corporate board and senior management teams with a collaboration and process management tool, our emphasis on structured development and security has always been paramount.
PLATFORM ARCHITECTURE
Role-based access control for users involved in multiple studies via master dashboards, calendars and reporting
Multiple workspace capabilities for controlled studies to protect unblinded data
Multi-factor authentication upon log-in, or single sign in for organizational access
Third-party audits of information security and regulatory compliance
INFORMATION SECURITY
SOC 2 certification independently certifies that our internal and external controls are focused on enterprise grade “Security, Privacy, Availability, Process Integrity and Confidentiality"
Partnership with Google Cloud® means a high-level of security protocols, encryption, intrusion detection, and physical data center protection
“Zero Trust” principles for forward-thinking, world-class security
Regular penetration and phishing testing from independent third parties
Two-factor authentication is standard, with the option to have two-factor authentication at a customer account level or an individual level
REGULATORY COMPLIANCE
Compliant platform that meets the requirements of 21 CFR Part 11, Annex 11, GDPR and HIPAA
Development practices follow SOPs that allow our life sciences clients to comply with applicable regulations
Independent, external validation of compliance with ongoing software updates by team
5. IMPLEMENTATION & SUPPORT
Cloud Concinnity® transforms clinical trial oversight work in powerful ways from day one. Our software team has built an intuitive, user-friendly platform that makes it easy to get up and running. From set-up to onboarding to training to ongoing support, you will find our customer success team at the ready and committed to your success from start to finish.
SET-UP & ONBOARDING
We offer strategy and success planning, onboarding and configuration support, a dedicated service manager, and online training resources. Before a study begins, we configure Cloud Concinnity® to your specific requirements. We obtain information about your study, your members and the way you’ve organized your data to set up users and permissions. We deliver your oversight committee workspace(s) with a labeling and folder structure that organizes your study and meeting documents.
TRAINING
We provide custom training tailored to the roles and needs of your users. Our Customer Success Team remains available throughout the study to provide additional training and assistance as needed. Our Customer Success Team will train your Administrators to have the knowledge and tools to support continuous process improvement as your study proceeds.. We recognize and appreciate that becoming expert software users is not a priority for all oversight committee members. We designed member training with this in mind, with a focus on clear communication, easy-to-use documentation, and email directions for initial sign-in and coordination with the member’s calendar. Comprehensive personal training sessions, conducted on Zoom, are tailored for the user’s role, meeting each member’s specific needs.
SUPPORT
We are dedicated to providing a wonderful experience with Cloud Concinnity for every customer and every user of our platform. Support is available 24/7 so your users can get the help they need, when they need it, no matter the time zone or hour. Support can be accessed through the Cloud Concinnity Support Center online, or via telephone by calling our Help Desk, which is backed by our on-call support team. Customer success representatives can provide additional one-to-one training or guidance for the duration of the study in response to changes in your study, staff or users.
BRINGING GOOD PROCESS TO CLINICAL TRIAL OVERSIGHT
Oversight committees deserve the best possible software tools to address the challenges of the future. As the clinical trial ecosystem moves further into the digital era, trust Cloud Concinnity® to deliver facilitated access, controlled process, and transparent outcomes that support oversight committees and patient safety while bringing life saving therapeutics to market.