4 Critical Takeaways from AstraZeneca and its DSMB
News that a Data Safety and Monitoring Board (DSMB) overseeing AstraZeneca’s Covid-19 vaccine trial publicly challenged the company’s drug efficacy reporting has been a gut punch to the entire clinical trial ecosystem.
In a world clamoring for faster and faster vaccine development, we are seeing spectacular success in getting life saving drugs to market. We are also seeing a system pushed to its limits. Developing multiple safe, effective vaccines in under a year is incredibly impressive. Distributing hundreds of millions of doses is equally amazing. At the same time, the stress and pressure of moving quickly while also grappling with rapidly evolving communication requirements and procedural adaptations has introduced a host of new challenges at every point in the drug development process.
Critical independent trial oversight, the kind provided by DSMBs, historically progresses over months and years. That luxury of time to act, react and communicate more deliberately has disappeared.
Whatever the multiple and varied root causes of the current clinical trial crises are, they shine a bright light on what’s critical and essential in good clinical trial oversight. We are seeing in real time just how important DSMBs are. We are also seeing that communication and process norms that have worked for decades need to evolve, change, and adapt to the new normal.
Every clinical trial stakeholder who is involved in the regulatory and independent oversight committee processes, from sponsors, to CROs, to DSMBs, can seize this moment to look at communications, processes, and systems.
1. TAKE A HARD LOOK AT COMMUNICATIONS
It’s not the people. It’s the processes. It’s the systems.
The vast majority of people in the clinical trial ecosystem — researchers, sponsors and independent bodies — approach their work with high scientific and ethical standards.
Mistakes can easily happen when processes and systems for coordinating and documenting oversight are stretched too thin, or when oversight tools are decentralized. Today most DSMBs are forced to rely on processes and systems for creating transparency, communications and reporting that are fragmented inside and outside of their organizations. Administrators can find themselves at the center of rules, communications and processes that are not optimized for speed and efficiency and increase risk for non-compliance or miscommunication.
Without an integrated set of information and communication tools, it’s impossible to know the status of oversight committees or to audit effectiveness in uncovering research issues or unintentional process violations. It’s a real threat to otherwise tight clinical trial processes.
2. CONSIDER HOW STANDARDIZED PROCEDURES IMPACT TRUST & SAFETY
Standard Operating Procedures (SOPs) have a critical role to play for DSMBs and the overall process of clinical trial oversight. Whether born of the charter and study protocol, the safety plan, or other documented sources, creating and following SOPs is the road to safe, speedy trials that inspire trust.
The challenge facing DSMBs and administrators is not so much knowing or developing these operational best practices, but implementing, tracking and reporting on them. Too many studies are still relying on manual processes or using multiple software tools that aren’t designed to work together. The results of these kinds of disconnected solutions are not pretty, as we are seeing.
The depth and breadth of these challenges demand simplification, centralization and automation. What is needed is a powerful process management engine that can drive an integrated process and provide visibility into current status and historical actions.
What’s needed is smarter technology to provide a process management backbone for the independent oversight mechanisms. The future success of clinical trials is dependent on digital transformation. It’s time for the oversight part of the clinical trial system to be supported by software as powerful as the oversight committees themselves.
3. SUPPORT YOUR OVERSIGHT COMMITTEES WITH SMART SOFTWARE
We believe in the power of smart technology to transform mission critical processes in service to a better world. Solving communication and process problems for oversight committees like DSMBs is our mission.
When we first launched Cloud Concinnity® as a governance, risk and compliance platform, it was used to solve a range of board and specialty governance management challenges. When COVID upended the world, it became the ultimate communication and process engine for DSMBs. We are passionate about helping DSMBs ensure patient safety while bringing life saving drugs to market.
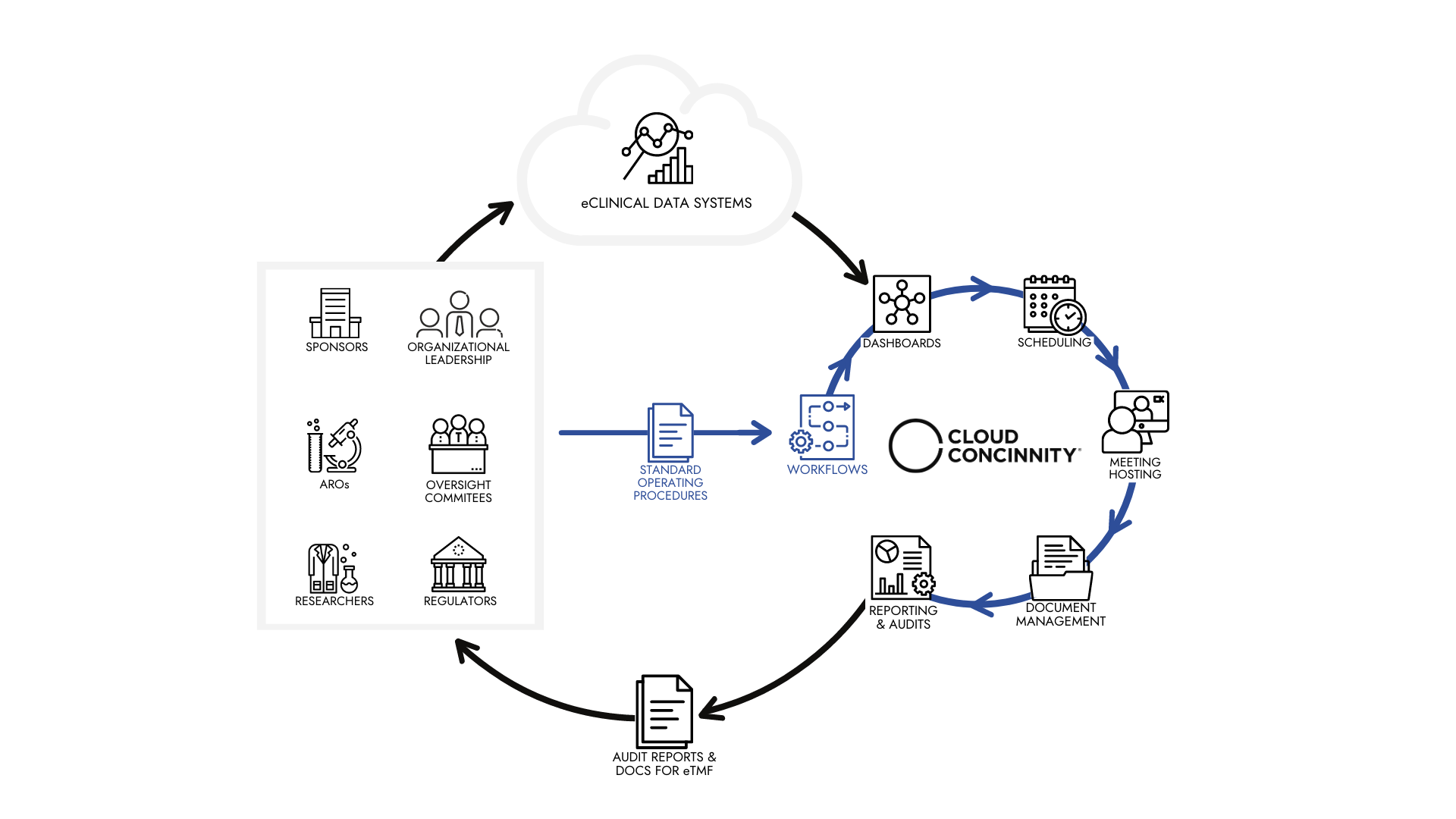
Cloud ConcinnityⓇ takes the work of an oversight committee and creates repeatable best practices, then instantly reports the status of the work and provides a comprehensive audit trail.
Think of Cloud ConcinnityⓇ as an eClinical solution built on a robust, integrated, cloud based governance process management engine. It provides a single, secure hub to organize, coordinate and document the end to end activities of the DSMB.
Whatever the appropriate processes are for any trial oversight committee, here is how Cloud ConcinnityⓇ helps improve the execution, documentation and visibility of SOPs:
SOPs for an oversight committee are broken down into tasks
Tasks are assigned to individuals by their roles
Execution of the tasks is tracked
All actions within the system are logged
Regular reporting of SOP status is visible as desired
Comprehensive audits are available in seconds, since all work is in Cloud Concinnity
4. ACT NOW
Today’s flurry of news is not just an urgent, pressing problem for the current round of vaccine development — it is a clarion call for every stakeholder in the clinical trial ecosystem moving forward.
Imagine the improved communications for DSMBs that begin using a single, secure cloud based hub to communicate. Imagine how clear and strong that message about the culture of respect and support for DSMBs will be sent. Imagine how sponsors can step up right now and show how fully they support their DSMBs by providing smart technology.
The problems flooding the news right now are preventable for those ready and willing to take action. Smart software solves communication problems. SOPs support safer, more trustworthy clinical trials.
We have the solution, ready to be deployed.
To learn more, explore our DMC & DSMB software.
For details, check out our Features & Benefits document.